Report Back from SABCS 2021: What’s the Latest in MBC Research?
According to Dr Stephanie Graff, a wonderful science translator and a past guest of this podcast,
“At every large oncology conference, a theme begins to emerge. This is typically driven by factors such as technological advances that shift research interests or the “me too” phenomenon in which drug and biotech companies start making drugs similar to ones that early studies suggest appear promising.”
With Dr Graff’s assistance we came up with three main themes at the latest San Antonio Breast Cancer Symposium
Theme 1 We know what we know.
A number of presented trial updates confirmed that data shown earlier was true and the drugs that had been expected to work really work. These trial updates provided long-term data on patient experience, drug safety, and outcomes. The follow-up analyses also allowed researchers to look at smaller subsets of the . For example, researchers reported that in the MONALEESA02 trial, which studied letrozole with or without ribociclib in metastatic breast cancer, every subset benefitted from ribociclib.
In the KEYNOTE-355 study that looked at adding pembrolizumab (or Keytruda) to other treatments used for metastatic triple negative breast cancer, the longer-term follow-up data showed that the drug continued to work for those who had benefitted initially. This means that checkpoint inhibitors (and we will talk about them later) will continue to be a good option for many people with triple negative breast cancer. An update on the Destiny Breast 03 trial showed that a subset of patients with brain metastasis had a very good response rate to Trastuzumab deruxtecan (T-Dxd), which was expected.
Theme 2 New things are always on the horizon.
Neratinib shows promise in Her2 mutant breast cancer (which is surprisingly different from Her2 positive breast cancer). Datopotamab deruxtecan (Dato-DxD), the TROP2-directed antibody-drug conjugate (ADC), displayed strong and durable efficacy results in the form of overall response rates (ORRs) when treating patients with metastatic triple-negative breast cancer, according to cohort data from the phase 1 TROPION-PanTumor01 trial. IT created a lot of buzz and will be moving forward in many subsequent trials. Elacestrant, the first of many oral selective estrogen receptor degraders (SERDs) to hit the big stage, showed only a modest improvement in survival. Whether it'll get to the finish line first remains to be seen. This is an exciting dimension because we really haven't had new endocrine agents for almost 20 years.
Theme 3 how do we individualize our treatment to the specific patient and the specific time during their therapy?
A novel trial design used circulating tumor DNA to determine when to change therapy as a way to potentially outsmart chemotherapy resistance or early cancer growth was interesting, but the concept needs to be tweaked before it is ready for primetime. A liquid biopsy-based approach to treatment selection improved outcomes among patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Patients treated with an aromatase inhibitor plus ibrance who displayed a rising ESR1 mutation in their blood prior to disease progression doubled their median PFS if they switched to fulvestrant plus ibrasnce rather than remaining on the same regimen, results of the randomized phase 3 PADA-1 trial showed.
“PADA-1 is the first trial to demonstrate that, in most patients, resistance-associated mutations in the estrogen receptor gene can be detected and targeted before tumor progression, The trial suggests a statistically and clinically significant benefit when fulvestrant is used during this very new window of opportunity.”
Use of genomics to guide treatment selection improved outcomes for certain patients with metastatic breast cancer, according to the randomized phase 2 SAFIR02-BREAST results Multigene genomic sequencing has been widely adopted. However, it had not been established whether multigene sequencing improved outcomes for patients with metastatic breast cancer. The general implication of this study is that precision medicine can improve patient outcome if it is interpreted with the right tools”
Transcript
This transcript has been edited and condensed for clarity.
What I want to focus on today is the few main areas that we'll start with HER2+ disease, where for me, one of the biggest result was a trial called Destiny Breast 03 which looked at the new antibody, drug conjugate T-DXd compared to TDM-1 and show that this is substantially superior at a second/ third line setting. But there are also other new drugs coming through, as we speak. We’ll have to work out how we best integrate them into the patient pathways.
In triple negative breast cancer we have more substantial data on the role of immune therapy in the first-line setting in a trial we have long waited for its final readout in terms of overall survival. It is called KEYNOTE-355, and it has now given us further evidence, that the incorporation of immune therapy in a first-line treatment setting substantially improves overall survival in the group of patients with PD-L1 positive tumors. We've also learned a little bit more about who the patients are, who might benefit from immunotherapy, which we are very happy to share with you. The second really exciting development in triple negative breast cancer, but we can also discuss later on how this will go to other subgroups as well, is the emergence of new antibody drug conjugates. So we'll talk a little bit about what an antibody drug conjugate is and how they work. Many of you are aware of Sacituzumab but also T-DXd alone or in combination with immune therapy. We've been seeing the emergence of a new subgroup. You've all heard of HER2 low disease. This is what I would call a therapeutic subgroup. And we'll explain a little bit where this comes from and what I hope to see in the next six to 12 months for this group of patients. For hormone driven, breast cancer ER+ disease we have seen really important data that provides further evidence that the incorporation of CDK4/6 inhibitors can improve, not just short-term outcome, but also long-term outcomes and substantially improve overall survival. Whether it's with aromatase inhibitors or fulvestrant. We are learning more and more what changes occur in tumors with resistance to CDK4/6 inhibitors. I've learned some interesting data about mutations in the cancer and how we can possibly tackle them therapeutically going forward. We have seen the first positive trial of a new group of drugs for hormone-driven breast cancer called SERDs or oral SERDs drugs. And again, that gives me a lot of hope, as we will use the data to move these drugs to earlier lines of treatment, where they will probably make more of a clinical benefit.
(From the transcript, Professor Schmid)
Now, if I may start with HER2+ breast cancer, and again, I have to apologize. This is very much from a European perspective. I know that we treat slightly differently on the different sides of the Atlantic. When it comes to metastatic disease recurrence in most countries in the world for many years now, the standard in the first line and the first treatment’s setting has been giving a chemotherapy group called taxanes in combination with two drugs that target HER2, which is the driver of these HER2+ breast cancers: trastuzumab and pertuzumab, In the second line setting normally it reverts to TDM-1
(From the transcript, Professor Schmid)
An interesting study was presented at ASCO a few months ago, slightly different strategy, because most patients with HER2+ breast cancer in Europe at least will initially be started on a chemotherapy with HER2 targeted therapy. But this study that was conducted in China, looked at either hormone therapy plus HER2 targeted therapy or chemotherapy plus HER2 targeted therapy in patients with HER2+ breast cancer, but also hormone receptor positive disease.
And what was interesting to see, that it's obviously a milder strategy, giving hormone therapy rather than chemotherapy. And in this particular trial the results were very similar in terms of how long the cancer was controlled, which is what we describe as progression-free survival, but also how long patients are able to live with their disease.
And that is an interesting result. We should use it to think about our strategies and do a little bit more work in the context of our current treatment standards. An interesting subgroup analyses show that patients, who have earlier disease recurrence from primary breast cancer treatment probably benefit from chemotherapy. On the other hand, the longer it takes before breast cancer comes back patients benefit from the combination of HER2 targeted therapy and hormone therapy, according to this trial. So certainly warrants further investigation (From the transcript, Professor Schmid)
When it comes to the second line setting, patients would routinely get in Europe TDM-1 as second line therapy. In this setting, on average this treatment gives patients benefit of around nine months.
(From the transcript, Professor Schmid)
Again, what I want to share with you is really how the treatment has changed over the last two or three years. One of the big changes is the introduction of Trastuzumab Deruxtecan, a new antibody drug conjugate that has substantially more activity with a progression-free survival of nearly 20 months.
And I want to talk a little bit about what these antibody drug conjugates are in their structure. This antibody drug conjugate is supposed to target on a surface protein in cancer cells. And this antibody has, through linker protein, a very powerful chemotherapy drug glued to it. Most antibody drug conjugates are very similar in their biology. (From the transcript, Professor Schmid)
What we are learning is that there are substantial differences between different ADCs. So we can't just say an ADC that targets HER2 are all the same. They can be massively different in terms of the antibody, in terms of how this drug is glued to the antibody drug conjugate and that will define whether that gets released somewhere in the periphery or the very target in the cancer tumor, but also in terms of the chemotherapy drug that is bound to the ADC. In the past it was thought, the was ADC works: it binds to the cancer cell then that gets internalized into the inside of the cell. The antibody gets broken down. The chemotherapy drug gets released and it can ultimately start killing cancer cells. What we've learned very recently is that many of these new ADCs (antibody drug conjugates) have an additional way of working. And this is what we call a bystander effect. Bystander effect means that the drug isn't just staying in this cell where it initially binds to, but it can also destroy and tackle some of the cancer cells that are immediately around there. And this happens through two mechanisms. The chemotherapy drug can get back out of the cell.
Some of the drugs release toxic molecules just before they get taken up in the cancer cells. Now, if you look at this new drug T-DXd compared to the TDM-1, the standard in a second line setting, you can see why these drugs are different, more drug-to-antibody-ratio and different chemotherapy drug linked to it.
But also most importantly, there's a substantial bystander effect.
(From the transcript, Professor Schmid)
The data we were waiting for a long time. and which are in my opinion groundbreaking and really changed the way we treat breast cancer. are from the Destiny Breast 03 trial. This is a trial of patients with metastatic HER2+ breast cancer. Patients were allowed to have brain metastases as long as they had been treated.
The patients were randomized between the old standard TDM-1 and the new drug T-DXd (Trastuzumab Deruxtecan). The trial was looking at how long we can ultimately control cancer, whether we can do this better with one or the other treatment. About 10% of patients had this treatment as the first treatment for metastatic disease.
40% as a second,and half of the patients as a third or subsequent line of therapy. The data were massively positive and are really changing the way we treat HER2+ breast cancer.
(From the transcript, Professor Schmid)
. If you look at the progression-free survival, the time the cancer was controlled with this treatment was doubling of the percentage of patients who had good disease control after a year. The median time that cancer was controlled was nearly four times higher, increasing the time that cancer was controlled from 7 months to 25 months. If you look at who are the patients who benefit from this therapy, you can see very easily see that everything seems to be confirming that T-DXD substantially better in all subgroups compared to TDM-1.
(From the transcript, Professor Schmid)
And looking at long-term outcome, looking at overall survival, patients who received T-DXD seem to be doing substantially better. And if you look at a simple and almost old-fashioned end point of response rate looking at whether the cancer actually shrinks, we see the response rate are nearly 80% with T-DXD compared to 34% with TDM-1 and with twice as many patients achieving a complete response, where no cancer is visible, but most importantly, only 1% of patients had failure of the treatment right at the beginning compared to 18% with TDM-1.
So really groundbreaking activity, that in my opinion makes this treatment a new standard of care in this set of patients.
(From the transcript, Professor Schmid)
Among the clinical problems we often have in management of patients with HER2+ breast cancer is that some of the treatments don’t tackle possible brain involvement very well. And so one of the analysis of the T-DXD study was to look at the group of patients who had brain involvement and those patients did equally well compared to TDM-1 with T-DXd (Trastuzumab Deruxtecan) as the rest of the group.
So we can clearly say this drug doesn't just work outside the brain. It also provides substantial benefit for patients with brain involvement, as you can also see from the response rates, which were twice as high with 64% compared to 33% with TDM-1. And those data compare very favorably with a second drug that has shown great promise in patients with brain involvement over the last two years, called Tucatinib. In HER2CLIMB study they offered Tucatinib to patients with brain metastases that were treated and stable, but also with brain metastases that were still active. Both groups of patients had had a clear benefit from the addition of Tucatinib to HER2 targeted therapy with similar benefits as we see for Trastuzumab Deruxtecan. So my conclusion here is, we have a new standard of care, but we also have now two very effective therapies that can help improve the control of brain involvement and outside the brain in patients with HER2+ breast cancer.
(From the transcript, Professor Schmid)
One of the important questions always, what is the price to pay for patients in terms of side effects? And there are subtle differences between T-DXd and TDM-1. TDM-1 has a little bit more increase in liver function tests versus T-DXd, a little bit more nausea and diarrhea which we have to learn to manage. TDM-1 has more thrombocytopenia, TDXd more decrease of white cell levels.
But ultimately these are all side effects we know how to manage. The one side effect we were a little bit more anxious about from the initial studies was something called pneumonitis. The pneumonitis rate was around 10% and it's still something we need to monitor patients very carefully. But with good management, most patients have mild side effects, well tolerated. I mentioned earlier that there are other ADCs( antibody drug conjugates) coming through.
(From the transcript, Professor Schmid)
For example, SYD985 which is not yet available, these are just clinical research data. The TULIP trial presented at the European conference in September-October time compared SYD985 with standard chemotherapy plus HER2 targeted therapy and patients with at least two lines of therapy.
(From the transcript, Professor Schmid)
And this drug improves PFS, not yet improves overall survival, but clearly it gives us hope that there are other treatment options beyond T-DXd.
(From the transcript, Professor Schmid)
Prior to the Destiny Breast 03 data most patients with HER2+ cancer would have received Trastuzumab and Pertuzumab in combination with taxanes as a first treatment, when that cancer started growing they would have been switched to TDM-1 and then had a third treatment T-DXd.
With the data I just shared with you this is now changing. Patients should now receive T-DXd much earlier as a second line therapy, but at the same time we need to see what we are doing after T-DXD. And one of the things we are working actively on in terms of research, is trying to understand - are those tumors after T-DXD still HER2 expressing? What's the mechanism of resistance to T-DXD which will allow us to tailor future strategies more effectively? Where are we going to head in the development with HER2+ breast cancer? And if I can just speculate where we may be in two or three years' time, the T-DXD drug is clearly at the moment one of the most effective drugs we have ever seen in this context.
And there are studies now ongoing that explore this drug as first-line therapy. And if you look at the activity we've seen in the second-third line setting, it is in my opinion, fairly likely that this drug may be superior in terms of efficacy to our current standards in the first line setting. But we obviously need to wait for the trial results for that, but we will also have to assess with the different treatment strategies, what impact they have on the quality of life of patients, because treatment strategies have different side effect profiles. We'll need to see in real life, how those treatments affect patients
(From the transcript, Professor Schmid)
We move on to triple negative breast cancer. Those tumors that neither have estrogen nor progesterone, so no hormone receptors and no HER2 receptors. When it comes to metastatic triple negative breast cancer, the shape and options changed over the last few years substantially and clearly improved what we could offer with the new standard of chemotherapy and immune therapy for patients with PD-L1 positive tumors.
PARP inhibitors in patients with BRCA germline mutations and otherwise single agent chemotherapy. And then in the second or third line setting a new targeted option really coming through, that isSacituzumab, a new antibody drug conjugate that provides clear patient benefits in this situation.
(From the transcript, Professor Schmid)
One of the challenges we've had with triple negative breast cancer, one of the reasons why progress probably took a little bit longer than, for example, in ER+ breast cancer is that it is a very heterogeneous disease. What does that mean? It's probably not one subtype of breast cancer, it's as we would say in England, a mixed bag of different subtypes of breast cancer that share one characteristic being that they don’t have hormone receptors and don't have HER2 receptors. So the different subtypes can be very, very different in their behavior, but also in response to different treatments. There have been many attempts to classify triple negative breast cancer based, for example, on gene expression profiling.
And whilst this has been helpful for us to understand the differences in the biology of triple negative breast cancer, therapeutically it's not really helpful. And therefore in the future we will probably describe triple negative breast cancers, as an example, this is a patient whose tumor is PD-L1 positive with SP142 assay of IC 3%, CPS score of 15, BRCA germline mutation drop too high, HER2 low, an antigen receptor free. And that will allow the oncologist to work out what are the best treatment strategies and also in which sequence should we give these treatments to patients.
(From the transcript, Professor Schmid)
And that will allow the oncologist to work out what are the best treatment strategies and also in which sequence should we give these treatments to patients. Now, the two areas that we have seen a lot of change in the last 12 months is immune therapy. We finally had survival data from the second big Phase 3 trial in this setting. Impassion 130 was the first , KEYNOTE 355 was the second. Very similar trials giving chemotherapy together with immune therapy as a first treatment to patients with metastatic triple negative breast cancer. And what we have previously learned is that the big subgroup of patients who have PD-L1 positive tumors benefit. We have now seen in both trials that the addition of immune therapy clearly extends substantially and in a meaningful way overall survival with both of those trials, but we've also seen that patients with PD-L1 negative tumors do not seem to benefit from this therapy.
(From the transcript, Professor Schmid)
There's an ongoing debate about those different PD-L1 tests. And I'm not going to go too much into detail because it can be confusing, but I just want to mention the 2 assays we use for triple negative breast cancer. One is called SP142, and the other 22C3. It comes down to looking at the right cutoffs. 22C3 with a cutoff of a 1% shows 80% are positive, but SP142 with cutoff of 1%, only 40% of patients are positive. And if you use a cutoff of 10 for the 22C3 assay again, only 40% are positive.
And this seems to be the group of patients who benefit. So it comes down to the small print, but you get a PD-L1 assay to work out whether immune therapy is the right therapy for you or not. We've also done additional work in trying to identify possible other subgroups that may or may not benefit from immune therapy by looking at the immune phenotypes, looking at molecular phenotypes. And the bottom line is whatever subtype of triple negative breast cancer it is, there is always a group of patients who have PD-L1 positive tumors, and that is the best marker to select patients for treatment with immune checkpoint inhibitors.
(From the transcript, Professor Schmid)
We also starting to understand what drives resistance to immune therapy. One of the factors I want to highlight here is angiogenesis, and that's the formation of blood vessels driven by the cancer. The reason why I mentioned that is we have drugs that target these blood vessels. There was a very small study presented at ASCO this year.
They gave a trifecta combination chemotherapy, immune therapy and anti-vascular drugs targeting these new blood vessels. The majority of patients had a substantial response to this therapy. This is not standard of care. This is not something we should do outside of trials, but this gives us hope that again, we can drive research in a different direction, hopefully improve the outcome of patients in this disease setting.
(From the transcript, Professor Schmid)
The second big area where we are making progress is antibody drug conjugates. So you heard about the biology of these drugs in HER2+ breast cancer. The same principle is applied in triple negative breast cancer, but we use different surface molecules to anchor these ADCs.
Many of those show early promise, some targeting something called Trop2 with a drug called Sacituzumabor or more recently Dato-DXd targeting HER2, targeting HER3 so there's a range of these new drugs coming through, which gives me a lot of hope and are likely to give patients more chances in the years to come The front runner here is clearly Sacituzumab, the drug that is already licensed and routinely used.
(From the transcript, Professor Schmid)
And this was based on the results of a trial that compared Sacituzumab with standard single agent chemotherapy in patients who had already had two lines of triple negative breast cancer, looking at progression-free survival and overall survival, the drugs substantially improved the progression-free survival.
It almost doubled overall survival in this heavily pretreated group of patients where it was in the past very difficult to make treatments work, but where we see now survival time twice as long as before. And most importantly, and again, coming back to an old-fashioned end point objective response, when we saw seven times higher response rate with Sacituzumab compared to standard chemotherapy.
Now, why is the response important? If patients have some, for example shortness of breath or cough because of lung involvement and if we just stabilize the cancer with the treatment and not make it shrink, the symptoms are not going to get better. So if you have a treatment that makes the cancer shrink in a third of patients compared to 5% of patients that clearly gives patients symptomatic benefit.
And we saw this in the quality of life data that showed a clear improvement for patients who received the new treatment compared to standard chemotherapy. And that improvement was carried on even into the next line of therapy. We saw some interesting biomarker data. Can we select patients based on the expression of Trop2, which is the target of the ADC. And the short answer is that it's not necessary because even if Trop2 is very low. Cancer patients still do better with this drug compared to standard chemotherapy.
(From the transcript, Professor Schmid)
There's also a second drug being developed that targets the same target Trop2, and this drug is called Dato-DXd and it's exciting and uses the same backbone the same biology as T-DXD I spoke about earlier in HER2+ disease in a small study, early data.
We see substantial activity again in patients with heavily pretreated, triple negative breast cancer. And there are two things that give me hope. The patients that have prior treatment Sacituzumab. So something that targets the same time. And we're still able to respond and patients who had prior treatment with a similar platform drug, which again, shows the patients can also still respond.
And that tells us a lot about the mechanisms of resistance. It gives us hope that we can give some of those treatments sequentially with good patient benefit. I said it at the beginning of.
(From the transcript, Professor Schmid)
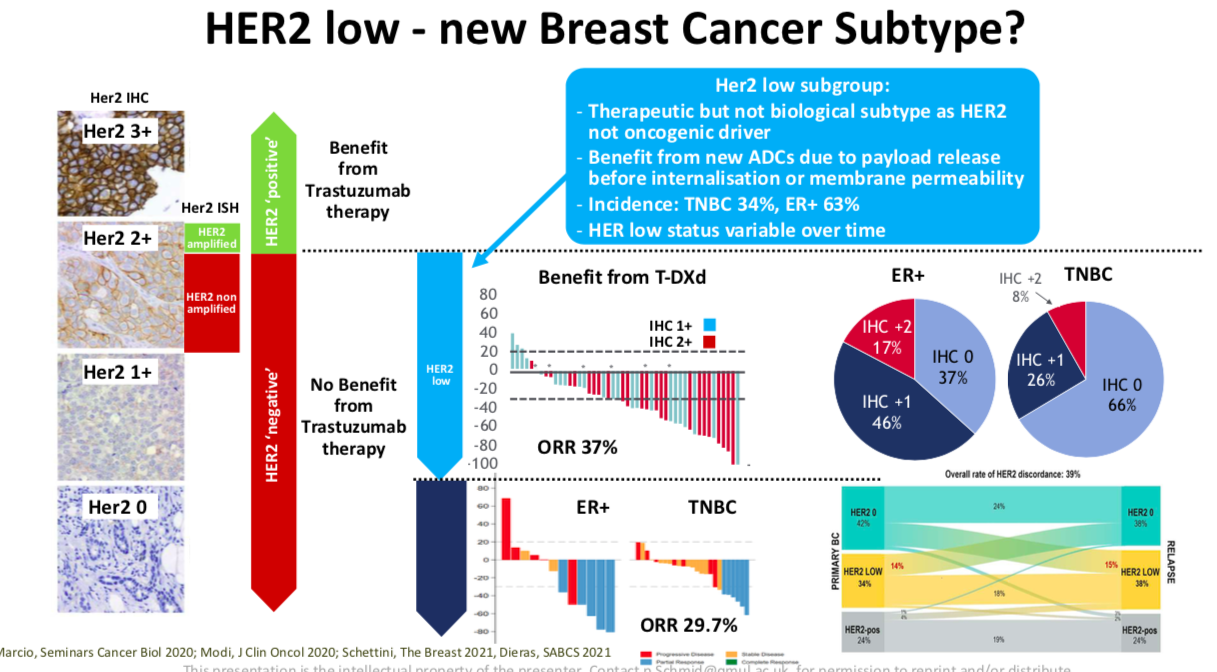
We also defining a new subtype of breast cancer called HER2 low disease. Now, going back in history 20 years ago, where we started really looking routinely on HER2+ breast cancer, the definition of HER2+ was based on how patients responded to trastuzumab therapy. Patients only benefited from trastuzumab if they had very high expression, which is called IHC three plus on a tumor biopsy. These patients would get a benefit from the trastuzumab therapy.
Whereas the patients with low expression would not. Now with these new drugs like T-DXd, that has the bystander effect, every single cell does not need to have high HER2 expression. We see interestingly enough now also activity with HER2 targeted drugs even in patients with only low HER2 expression.
And that's a huge group of patients, two thirds of patients with hormone receptor positive breast cancer or one third of patients with triple negative breast cancer. We will see patients with very, very low almost absent HER2 expression with some possible response to this therapy. We are eagerly awaiting the trial data in HER2 low patients. The Phase 3 data are expected to come in the next few months.
And I think that will help us to identify again, a new therapeutic although not biological subtype, with HER2 low disease. It gives us new treatment that gave me a lot of hope for patients.
(From the transcript, Professor Schmid)
Finally we are working on combining these new ADCs with immunotherapy.
You've seen the data with immunotherapy. You see the data with ADCs, but if you put those two drugs together, we have data from a small study we presented at ASCO earlier this year in patients who had low HER2, so they had low HER2 expression and were predominantly PD-L1 negative where immune therapy normally doesn't really work and practically all patients had some shrinkage. Two-thirds of patients had substantial shrinkage of the cancer, which gives me a lot of hope and we will be pursuing this and other studies to hopefully bring this to patients as the standard therapy. The key question for us in research to address over the next few months, is looking at the role of immune therapy in patients who have already received immune therapy in an early disease setting, we will have to work on these antibody drug conjugates
(From the transcript, Professor Schmid)
And we will get interesting data in the next 12 to 18 months on further data on DNA damage repair targeting drugs, PARP inhibitors, but also AKT inhibitors where another Phase 3 trial will read out in the next year.
(From the transcript, Professor Schmid)
Let me come finally to hormone driven breast cancer. where we had an exciting five years in terms of having really improved outcomes of patients substantially, for example, with incorporation of CDK4/6 inhibitors into treatment options, which are now the standard treatment as a first-line setting, either with Aromatase Inhibitors or with fulvestrant in patients who have resistance to Aromatase Inhibitors.
And the three areas where we had interesting data where one key question was “Can we achieve long-term improvement of outcomes, so increase overall survival benefit with CDK4/6 inhibitors. And the short answer is “Yes, we can”.
(From the transcript, Professor Schmid)
And I will share the data with you. We have also been learning some of those emerging mechanisms of resistance to CDK4/6 inhibitors, which give us new treatment options, for example, targeting ESR1 mutations. Finally, we are looking strategies post-CDK4/6 inhibitors with oral SERDS being one of the groups of the drugs that get a lot of attention and give me hope going forward. If you look at the overall survival data first in patients whose tumors are already resistant to aromatase inhibitors, we have seen now consistently with a different CDK4/6 inhibitors in the different trials a clear improvement in overall survival. And if you look at the different subgroups of patients, where the patients get CDK4/6 inhibitors a little bit earlier or later, or have endocrine naive or hormond resistant disease, all patient groups seem to derive a very similar benefit. The only group of patients I want to single out,
is patients in one of the studies, those patients who received chemotherapy for advanced breast cancer and then only received the CDK4/6 inhibitors afterwards. They didn't seem to have quite as much of a benefit as patients who did not have prior chemotherapy for advanced disease, whether this is just play of chance in this trial or really telling us something about our biology, we can't say at this point in time. But it clearly just emphasizes the point that patients with hormone receptor positive breast cancer should receive endocrine therapy as the first treatment, wherever possible.
(From the transcript, Professor Schmid)
The data we were eagerly awaiting were the overall survival data in patients who received CDK4/6 inhibitors in combination with Aromatase Inhibitor in an earlier line of therapy. And we received data of the Monaleesa trial at the European conference and in San Antonio showing very clearly that patients will live longer if they get CDK4/6 in combination with hormone therapy and the benefit increases a year by year, at a four or five, six year survival data were substantially improved with the additional CDK4/6 inhibitors. And all patients seem to benefit equally, even patients who had chemotherapy prior to this in the early disease setting will derive a benefit.
(From the transcript, Professor Schmid)
What about when patients receive CDK4/6 inhibitors, what do we know about resistance? One of the interesting studies looked at genetic changing in the cancer induced by CDK4/6 inhibitors. There are clearly differences in genetic changes prior to treatment with CDK4/6 inhibitors and after CDK4/6 inhibitor therapy. And the differences are predominantly two areas, where one is in the area called ESR1. And many of you have heard of ESR1 mutations. ESR1 is the chain that encodes the estrogen receptor, our treatment target, and you can see substantially more mutations occurring in patients who have been on CDK4/6 inhibitor. It's an induced mutation and we got new drugs that target these mutations called SERDs. These are hormone therapies that don’t just block estrogen receptor, but they basically destroy estrogen receptor. So it's not just taking fuel away from an engine, it’s blowing up the whole engine and taking it out of the equation. And that's why this is an important mechanism of resistance because we can use it therapeutically. The second area that's emerging is something on chromosome 12 where we see change in gene called MDM2. And again, we have inhibitors in preclinical models that may hopefully over time give us new strategies of the treatment
(From the transcript, Professor Schmid)
. A really interesting study by the French group in exactly the same setting. So this was a study where patients received hormone therapy Aromatase Inhibitors combined with CDK4/6 inhibitors. After that, the group monitored patients regularly with a liquid biopsy looking for possible emerging mutations.
Genetic changes in the blood were targeting ESR1, and they looked at patients where these ESR1 were occurring, but where the cancer was still stable. The group was trying to answer a question if you could at that moment make switch in terms of the therapy, keeping CDK4/6 inhibitors, e.g. palbociclib, but changing the hormone therapy backbone away from AI (letrazole). To fulvestrant, which is another SERD compound that can remove this mutated receptor. The data I think are fantastic, are really exciting, really intriguing. We, by simply switching the hormone therapy before patients progress but if they already have this emerging ESR1 mutation, we could control the disease twice as long as we did before.And this isn't just down to giving the extra drug, it's down to using it at the right time when those mutations are emerging.
(From the transcript, Professor Schmid)
And there's a whole area at the moment, going into these new hormonal therapies. And these are called SERDs. As I mentioned before, they selectively destruct the estrogen receptor, but there are also other drugs SERM, SERCA, CERAN, PROTAC you will hear a lot about. They all share a few interesting characteristics that give me a lot of hope.One is their active after fullvestrant or active after CDK4/6 inhibitors, but most importantly, the work in patients in tumors with ESR1, but also without ESR1 mutations. And when we looked at some of the early data coming out, we saw encouraging activity with quite a few of those drugs.
(From the transcript, Professor Schmid)
The first Phase 3 data came out at San Antonio just over a month ago from the Emerald study with a drug called Elacestrant.
And this is a drug that was used in patients who already had a couple of lines of hormone therapy and all patients had already progressed on prior CDK4/6 inhibitors. So this is a group of patients where hormone therapy was not easy to still provide a benefit, but what was important to see when Elacestrant, the new third compound was compared with aromatase inhibitors or Fulvestrant, it clearly improved the time cancer was controlled, especially in patient with ESR1 mutations.
(From the transcript, Professor Schmid)
So if I may summarize just briefly in HER2+ disease we have a new standard of care moving forward very rapidly, called T-DXd, a new antibody drug conjugate that showed superior efficacy compared to TDM-1 in terms of progression-free survival. We are looking into other new drugs SYD985 and Pyrotinib just to mention, for HER2+ positive in phase three trials, but we will have to work out over the months and years to come how best to incorporate them possibly into treatment strategies.
In triple negative breast cancer, immune therapy is clearly standard of care in the first line setting in patients with PD-L1 positive tumors.
Based now on the overall survival data from KEYNOTE355, we show substantial improvement with different chemotherapy backbones, and we're learning more and more, how best to select patients for immune therapy benefits that seem to work across different molecular and immune subtypes of triple negative breast cancer
Antibody drug conjugates are for me for the next few years probably the most exciting developments. Sacituzumab is already standard of care in triple negative breast cancer for patients with at least two lines of therapy. T-DXd and Dato-DXd are emerging, even in triple negative breast cancer alone or in combination with immune therapy.
We spoke about how HER2 low disease is an emerging therapeutic but not biological subtype of breast cancer.
In ER+ breast cancer we finally have seen long-term survival improvement with CDK4/6 inhibitors in both sensitive and resistant disease. We are gradually understanding better the mutational profiles that are emerging in patients with resistance and can switch the endocrine backbone there. For example, in patients with ESR1 mutations to tailor therapies better, we are also seeing that oral SERD compounds are working.
We have a proof of principle and we will have to work out over the next few years where best to use them. And I would expect them to move into earlier lines. So we have overall made a lot of progress that gives me hope. We're clearly not where we need to be. Where we need to be at some point is that we can cure routinely patients with metastatic breast cancer in all subtypes.
And that's still not routinely possible, but we are making rapid progress with new treatments, with new groups of drugs that are more targeted that are better tolerance. And also improve long-term outcome of patients. I look forward to discussing these advances.
(From the transcript, Professor Schmid)
Want more?
Check out our past Road to a Cure episodes to learn about the latest MBC research from our guests, renowned clinicians and researchers, featured in this episode.






































Over the last 20 years, advances in HER2 targeting treatments, antibody-drug conjugates (ADCs), immune checkpoint inhibitors (ICIs), and cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6is) have prolonged survival and changed the breast cancer treatment landscape. Success leads to more questions about optimal drug sequencing, mechanisms of resistance, and how to overcome that resistance. Let’s ask Dr. Stephanie Graff to help us tackle these topics and more.